Published in Soft Matter, 2024
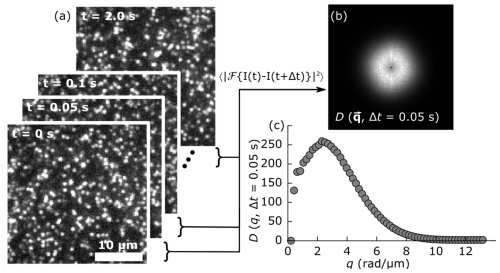
DNA, which naturally occurs in linear, ring, and supercoiled topologies, frequently undergoes enzyme-driven topological conversion and fragmentation in vivo, enabling it to perform a variety of functions within the cell. In vitro, highly concentrated DNA polymers form entanglements that yield viscoelastic properties dependent on the topologies and lengths of the DNA. Enzyme-driven alterations of DNA size and shape therefore offer a means of designing active materials with programmable viscoelastic properties. Here, we incorporate multi-site restriction endonucleases into dense DNA solutions to linearize and fragment circular DNA molecules. We pair optical tweezers microrheology with differential dynamic microscopy and single-molecule tracking to measure the linear and nonlinear viscoelastic response and transport properties of entangled DNA solutions over a wide range of spatiotemporal scales throughout the course of enzymatic digestion. We show that, at short timescales, relative to the relaxation timescales of the polymers, digestion of these ‘topologically-active’ fluids initially causes an increase in elasticity and relaxation times followed by a gradual decrease. Conversely, for long timescales, linear viscoelastic moduli exhibit signatures of increasing elasticity. DNA diffusion, likewise, becomes increasingly slowed, in direct opposition to the short-time behavior. We hypothesize that this scale-dependent rheology arises from the population of small DNA fragments, which increases as digestion proceeds, driving self-association of larger fragments via depletion interactions, giving rise to slow relaxation modes of clusters of entangled chains, interspersed among shorter unentangled fragments. While these slow modes likely dominate at long times, they are presumably frozen out in the short-time limit, which instead probes the faster relaxation modes of the unentangled population.
Download here